Sulfur is a component of many common minerals such as galena pbs gypsum caso 4 2 h 2 o pyrite fes 2 sphalerite zns or fes cinnabar hgs stibnite sb 2 s 3 epsomite mgso.
Sulfur phase at room temperature.
At room temperature rhombic sulphur is more stable and on heating it passes on to monoclinic sulphur which exists within a range of temperature and pressure hounded by pqr figure.
The region labeled i a solid region is α sulfur.
A room temperature superconductor is a material that is capable of exhibiting superconductivity at operating temperatures above 0 c 273 k.
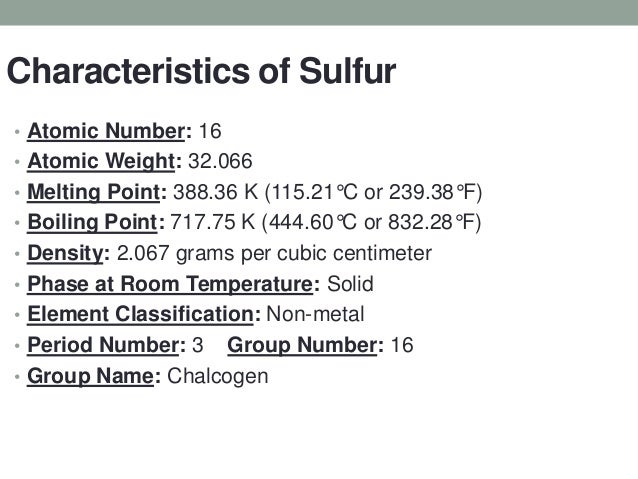
Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature.
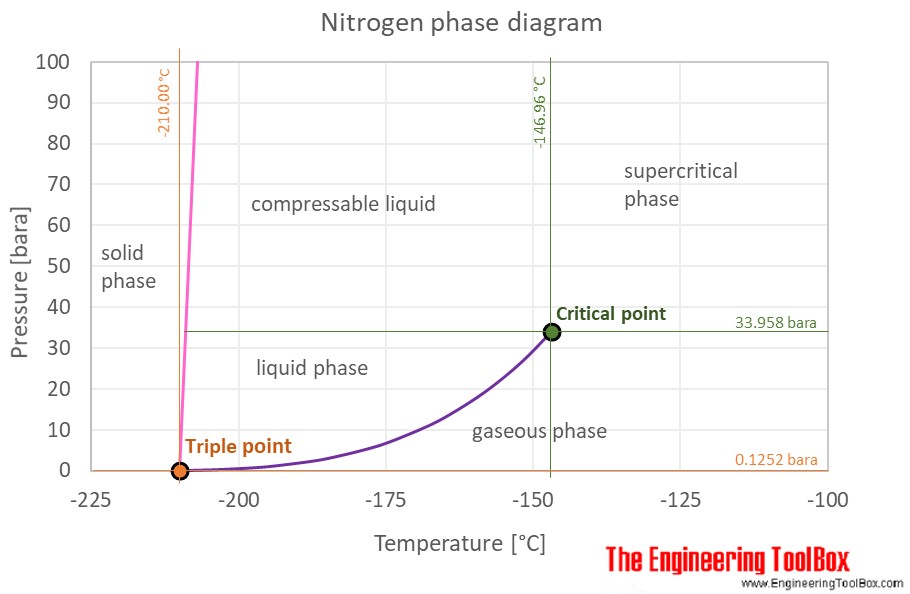
A phase diagram for sulfur is shown below.
Sulfur can exist as a gas a liquid or as one of two solid states.
Sulfur the tenth most abundant element in the universe has been known since ancient times sometime around 1777 antoine lavoisier convinced the rest of the scientific community that sulfur was an element.
The temperature at which rhombic sulphur passes on to the monoclinic form is 95 5 0 c.
1 0 atm at 25 c.
Properties sources and uses of the element sulfur and various compounds including sulfur hexafluoride and sulfur trioxide.
A metastable β sulfur phase stabilized at room temperature during cycling of high efficiency carbon fibre sulfur composites for li s batteries c.
Relative atomic mass the mass of an atom relative to that of.
In a high pressure study at ambient temperatures four new solid forms termed ii iii iv v have been characterized where α sulfur is form i.
Specifically sulfur is an orthorhombic crystal solid and can be recognized by its bright yellow color.
Phase at room temperature.
This is known as the transition temperature for rhombic sulphur to get.
While this is not strictly room temperature which would be approximately 20 25 c 68 77 f it is the temperature at which ice forms and can be reached and easily maintained in an everyday environment.
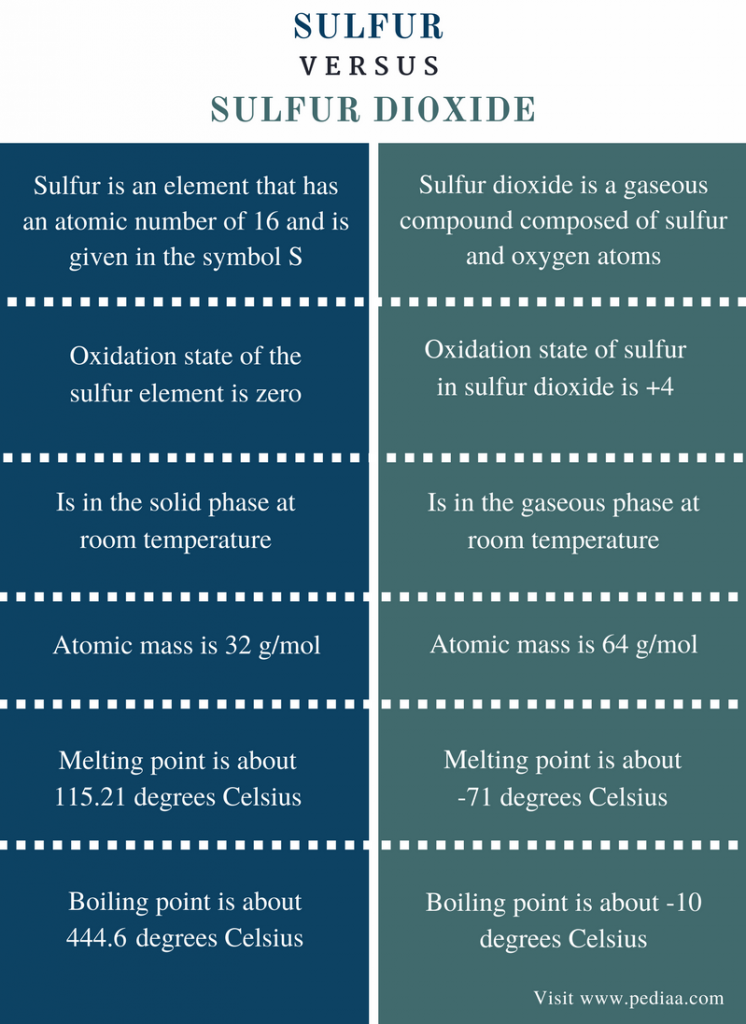
Sulfur is a solid at room temperature.
A 2013 1 13089 doi.
The pressure temperature p t phase diagram for sulfur is complex see image.
Sublimation the transition of a substance directly from the solid to the gas phase without passing through a liquid phase.
What is are the thermodynamically stable phase s of sulfur at room temperature and pressure i e.
High pressure solid allotropes.